Bictegravir 1611493-60-7
Tsanangudzo
Bictegravir chinyorwa, chine simba inhibitor yeHIV-1 integrase ine IC50 ye7.5 nM.
In Vitro
Bictegravir (BIC) inovharisa basa rekutamisa tambo neIC50 ye7.5± 0.3 nM.Zvinei nekudzivirirwa kwayo kwe strand transfer chiitiko, Bictegravir inhibitor isina kusimba ye3.'-kugadzirisa basa reHIV-1 IN, ine IC50 ye241±51 nM.Bictegravir inowedzera kuunganidza 2-LTR madenderedzwa ~ 5-fold maererano nekutonga-kunyombwa uye inoderedza huwandu hwechokwadi hwekubatanidza zvigadzirwa mumasero ane utachiona ne100-fold.Bictegravir inodzivirira kudzokororwa kweHIV-1 mune ese MT-2 uye MT-4 maseru ane EC50s e1.5 uye 2.4 nM, zvichiteerana.Bictegravir inoratidza zvine simba antiviral mhedzisiro mune ese ekutanga CD4+ T lymphocytes uye monocyte-inotorwa macrophages, ine EC50s ye1.5±0.3 nM uye 6.6±4.1 nM, zvichiteerana, izvo zvinofananidzwa nemaitiro anowanikwa muT-cell mitsara[1].
MCE haina kuzvimiririra yakasimbisa chokwadi chenzira idzi.Ndedzekureva chete.
| Nhamba yeNCT | Sponsor | Condition | Zuva Rokutanga | Phase |
| NCT03998176 | Yunivhesiti yeNebraska | Giriyedhi Sayenzi | HIV-1-utachiona | Gumiguru 9, 2019 | Chikamu chechina |
| NCT03789968 | Thomas Jefferson University | Yunivhesiti yeMaryland, College Park | Indiana University Hutano | The Brooklyn Hospital Center | University of Illinois kuChicago | Nova Southeastern University | University of California, San Francisco | HIV+AIDS | Gunyana 1, 2019 | |
| NCT04249037 | Yunivhesiti yeColorado, Denver | Giriyedhi Sayenzi | HIV+AIDS | Kurume 1, 2020 | Hazvigoneke |
| NCT04132674 | Vancouver Infectious Diseases Center | Human Immunodeficiency Virus I Infection | Kushandisa Zvinodhaka | Mbudzi 26, 2018 | Chikamu chechina |
| NCT04054089 | Cristina Mussini | Yunivhesiti yeModena uye Reggio Emilia | Hutachiona hweHIV | Gunyana 2019 | Chikamu chechina |
| NCT04155554 | Azienda Ospedaliera Universitaria Senese|Catholic University of the Sacred Heart|Ospedale Policlinico San Martino|Azienda Ospedaliera San Paolo|Ospedale Amedeo di Savoia | HIV-1-utachiona | Ndira 29, 2020 | Phase 3 |
| NCT02275065 | Gilead Sciences | Hutachiona hweHIV-1 | Gumiguru 2014 | Phase 1 |
| NCT03711253 | Yunivhesiti yeSouthern California | Acute HIV Infection | Gumiguru 14, 2019 | Chikamu chechina |
| NCT02400307 | Gilead Sciences | HIV | Kubvumbi 17, 2015 | Phase 1 |
| NCT03499483 | Fenway Community Health | Kudzivirira HIV | Ndira 24, 2019 | Chikamu chechina |
| NCT03502005 | Midland Research Group, Inc.|Gilead Sayenzi | Human Immunodeficiency Virus | Kurume 1, 2018 | Chikamu chechina |
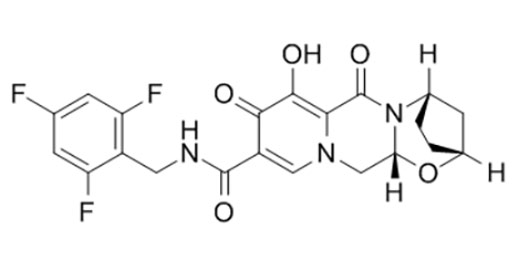
Chimiro chemakemikari





Proposal18Quality Consistency Evaluation mapurojekiti akatenderwa4,uye6mapurojekiti ari pasi pekubvumidzwa.

Yepamberi yepasirese yemhando manejimendi system yakaisa hwaro hwakasimba hwekutengesa.

Kutariswa kwemhando yepamusoro kunofamba kuburikidza nehupenyu hwese kutenderera kwechigadzirwa kuti ive nechokwadi chemhando nekurapa.

Professional Regulatory Affairs timu inotsigira zvinodiwa zvemhando panguva yekushandisa uye kunyoreswa.


Korea Countec Bottled Packaging Line


Taiwan CVC Bottled Packaging Line


Italy CAM Board Packaging Line

German Fette Compacting Machine

Japan Viswill Tablet Detector

DCS Control Room





