Dabigatran Etexilate Mesylate
Tsanangudzo
Dabigatran etexilate mesylate (BIBR 1048MS) chigadzirwa nemuromo chinoshanda cheDabigatran.Dabigatran etexilate mesylate ine anticoagulant mhedzisiro uye inoshandiswa kune prophylaxis yevenousthromboembolism uye sitiroko nekuda kweatrial fibrillation.
Background
Tsanangudzo: IC50 Kukosha: 4.5nM (Ki);10nM(Thrombin-induced platelet aggregation) [1] Dabigatran inodzokororwa uye inosarudza, yakananga thrombin inhibitor (DTI) iri kuenderera mberi nekiriniki yekuvandudzwa seyemuromo inoshanda prodrug,dabigatran etexilate.in vitro: Dabigatran yakasarudzika uye inodzoreredza inhibited thrombin yemunhu (Ki: 4.5 nM) pamwe nethrombin-induced platelet aggregation (IC(50): 10 nM), asi ichiratidza kusaita inhibitory kune mamwe maplatelet-stimulating agents.Thrombin generation in platelet -Poor plasma (PPP), yakayerwa seye endogenous thrombin inogona (ETP) yakavharidzirwa kusungwa-kutsamira (IC (50): 0.56 microM).Dabigatran yakaratidza concentration-dependent anticoagulant effects mumhando dzakasiyana-siyana mu vitro, kaviri iyo activated partial thromboplastin nguva (aPTT), prothrombin nguva (PT) uye ecarin clotting nguva (ECT) muPPP yevanhu pazvikamu zve 0.23, 0.83 uye 0.18 microM, maererano [ 1].in vivo: Dabigatran yakawedzera muyero wePTT-zvichienderana mushure mekutonga kwemutsinga mumakonzo (0.3, 1 uye 3 mg / kg) uye rhesus tsoko (0.15, 0.3 uye 0.6 mg / kg).Dose- uye nguva-inotsamira anticoagulant mhedzisiro yakaonekwa ne dabigatran etexilate inopihwa nemuromo kumakonzo anoziva (10, 20 uye 50 mg / kg) kana rhesus tsoko (1, 2.5 kana 5 mg / kg), ine yakanyanya mhedzisiro inoonekwa pakati pe30 ne120 min mushure mekutonga, zvichiteerana [1].Varwere vanobatwa ne dabigatran etexilate vakawana mashoma eschemic sitiroko (3.74 dabigatran etexilate vs 3.97 warfarin) uye vashoma vakasanganiswa intracranial haemorrhages uye haemorrhagic sitiroko (0.43 dabigatran etexilate vs 0.99 warfarin) pa1020 yemakore.Muedzo wekiriniki: Kuongorora kwePharmacokinetics uye Pharmacodynamics yeOral Dabigatran Etexilate muHemodialysis Varwere.Phase1
Storage
| Upfu | -20°C | 3 years |
| 4°C | 2 years | |
| In solvent | -80°C | 6 mwedzi |
| -20°C | 1 mwedzi |
Clinical Muedzo
| Nhamba yeNCT | Sponsor | Condition | Zuva Rokutanga | Phase |
| NCT02170792 | Boehringer Ingelheim | Hutano | Kukadzi 2001 | Phase 1 |
| NCT02170974 | Boehringer Ingelheim | Hutano | Chikunguru 2004 | Phase 1 |
| NCT02170831 | Boehringer Ingelheim | Hutano | Chivabvu 1999 | Phase 1 |
| NCT02170805 | Boehringer Ingelheim | Hutano | Kubvumbi 2001 | Phase 1 |
| NCT02170610 | Boehringer Ingelheim | Hutano | Kurume 2002 | Phase 1 |
| NCT02170909 | Boehringer Ingelheim | Hutano | Zvita 2004 | Phase 1 |
| NCT02171000 | Boehringer Ingelheim | Hutano | Kubvumbi 2005 | Phase 1 |
| NCT02170844 | Boehringer Ingelheim | Hutano | Chikumi 2004 | Phase 1 |
| NCT02170584 | Boehringer Ingelheim | Hutano | Ndira 2001 | Phase 1 |
| NCT02170935 | Boehringer Ingelheim | Venous Thromboembolism | Kubvumbi 2002 | Phase 2 |
| NCT02170636 | Boehringer Ingelheim | Hutano | Ndira 2002 | Phase 1 |
| NCT02170766 | Boehringer Ingelheim | Hutano | Gumiguru 2000 | Phase 1 |
| NCT02171442 | Boehringer Ingelheim | Hutano | Kubvumbi 2002 | Phase 1 |
| NCT02170896 | Boehringer Ingelheim | Hutano | Gumiguru 2001 | Phase 1 |
| NCT02173730 | Boehringer Ingelheim | Hutano | Mbudzi 2002 | Phase 1 |
| NCT02170623 | Boehringer Ingelheim | Hutano | Kukadzi 2002 | Phase 1 |
| NCT02170116 | Boehringer Ingelheim | Hutano | Mbudzi 1998 | Phase 1 |
| NCT02170597 | Boehringer Ingelheim | Hutano | Nyamavhuvhu 2003 | Phase 1 |
| NCT01225822 | Boehringer Ingelheim | Venous Thromboembolism | Mbudzi 2002 | Phase 2 |
| NCT02170701 | Boehringer Ingelheim | Venous Thromboembolism | Gumiguru 2000 | Phase 2 |
| NCT02170740 | Boehringer Ingelheim | Hutano | Mbudzi 1999 | Phase 1 |
| NCT02170922 | Boehringer Ingelheim | Hutano | Chikunguru 1999 | Phase 1 |
Chimiro chemakemikari
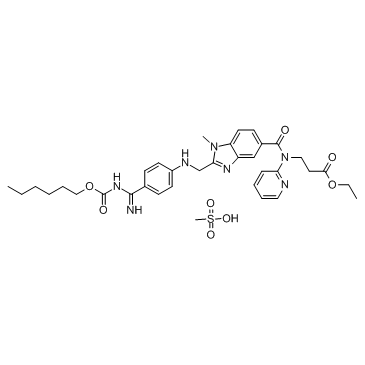





Proposal18Quality Consistency Evaluation mapurojekiti akatenderwa4,uye6mapurojekiti ari pasi pekubvumidzwa.

Yepamberi yepasirese yemhando manejimendi system yakaisa hwaro hwakasimba hwekutengesa.

Kutariswa kwemhando yepamusoro kunofamba kuburikidza nehupenyu hwese kutenderera kwechigadzirwa kuti ive nechokwadi chemhando nekurapa.

Professional Regulatory Affairs timu inotsigira zvinodiwa zvemhando panguva yekushandisa uye kunyoreswa.


Korea Countec Bottled Packaging Line


Taiwan CVC Bottled Packaging Line


Italy CAM Board Packaging Line

German Fette Compacting Machine

Japan Viswill Tablet Detector

DCS Control Room







