Doxycycline Hyclate
Background
Doxycycline hyclate mushonga unorwisa mabhakitiriya [1].
Doxycycline hyclate inobva kune tetracycline uye ine mabasa ekurwisa-kupisa uye antimicrobial. Doxycycline inhibits dengue virus replication in vitro nenzira inotsamira tembiricha. Iyo IC50 kukosha ndeye 52.3μM pa37°C uye 26.7μM pa40°C. Inodzivirira hutachiona hwedengue kuburikidza nekudzivisa iyo NS2B-NS3 serine protease yehutachiona. 60μM doxycycline inoderedza CPE yeDNEV2-ane utachiona masero [1].
Doxycycline inowanikwa kuva inhibitor yeMMP. Kurapa kweDoxycycline kunoderedza MMP-8 uye -9 mazinga uye kunodzivisa kutaura kwenyama MMP-2 uye MMP-9. Uyezve, kurapwa ne doxycycline kunoderedza zvakanyanya kuitika kwe intracranial aneurysms. Doxycycline yakave zvakare yakashumwa kuva anti-inflammatory agent inobva pakudzivisa kwayo matrix metalloproteinases. Mukuwedzera, doxycycline ine simba rekurwisa marariya rine IC50 kukosha kwe320nM pa96h in vitro [2, 3].
References:
[1] Rothan HA, Mohamed Z, Paydar M, Rahman NA, Yusof R. Inhibitory effect ye doxycycline against dengue virus replication in vitro. Arch Virol. 2014 Kubvumbi159(4):711-8.
[2] Maradni A, Khoshnevisan A, Mousavi SH, Emamirazavi SH, Noruzijavidan A. Basa rematrix metalloproteinases (MMPs) uye MMP inhibitors pane intracranial aneurysms: chinyorwa chekuongorora. Med J Islam Repub Iran. 2013 Nov;27(4):249-254.
[3] Draper MP, Bhatia B, Assefa H, Honeyman L, Garrity-Ryan LK, Verma AK, Gut J, Larson K, Donatelli J, Macone A, Klausner K, Leahy RG, Odinecs A, Ohemeng K, Rosenthal PJ, Nelson ML. In vitro uye in vivo antimalarial efficacies ye optimized tetracyclines. Antimicrob Agents Chemother. 2013 Jul;57(7):3131-6.
Tsanangudzo
Doxycycline (hyclate) (Doxycycline hydrochloride hemiethanolate hemihydrate), mushonga unorwisa mabhakitiriya, inoshandiswa nemuromo uye yakafara-spectrum metalloproteinase (MMP) inhibitor [1].
Clinical Muedzo
| Nhamba yeNCT | Sponsor | Condition | Zuva Rokutanga | Phase |
| NCT00246324 | Louisiana State University Hutano Sayenzi Center Shreveport|Biogen | Multiple Sclerosis | Zvita 2003 | Chikamu chechina |
| NCT00910715 | Yunivhesiti Medical Center Ljubljana | Erythema Chronicum Migrans | Chikumi 2009 | Hazvigoneke |
| NCT00243893 | Yunivhesiti yeCalifornia, San Francisco | National Institute of Neurological Disorders uye Stroke (NINDS) | Aneurysms | Arteriovenous Malformations | Chikunguru 2004 | Phase 1 |
| NCT00126399 | CollaGenex Pharmaceuticals | Rosacea | Chikumi 2004 | Phase 3 |
| NCT01318356 | Radboud University|ZonMw: Iyo Netherlands Organisation for Health Research and Development | Q Fever | Kuneta Syndrome, Chisingaperi | Coxiella Infection | Kubvumbi 2011 | Chikamu chechina |
| NCT00177333 | Yunivhesiti yePittsburgh | Kubvisa nhumbu, kuitiswa|Kurutsa | Gunyana 2005 | Chikamu chechina |
| NCT00007735 | US Department of Veterans Affairs|Pfizer|United States Dhipatimendi reDziviriro|VA Hofisi yeKutsvagisa neKuvandudza | Persian Gulf Syndrome | Mycoplasma Infections | Ndira 1999 | Phase 3 |
| NCT00351273 | Yunivhesiti yeSouth Florida | National Institute of Arthritis uye Musculoskeletal uye Skin Zvirwere (NIAMS) | Arthritis, Reactive | Reiter Chirwere | Chivabvu 2006 | Phase 3 |
| NCT00469261 | Careggi Hospital | Myocardial Infarction | Kuruboshwe Ventricular Remodeling | Chivabvu 2007 | Phase 2 |
| NCT00547170 | Yunivhesiti yePittsburgh | Tu Du Hospital | Endometritis | Ndira 2007 | Chikamu chechina |
| NCT01475708 | Yunivhesiti Medical Center Ljubljana | Lyme Borreliosis | Chivabvu 2011 |
|
| NCT01368341 | Morten Lindbaek|Norwegian Institute of Public Health|Sorlandet Hospital HF|Norwegian University of Life Sciences|Yunivhesiti yeOslo | Erythema Migrans | Erythema Chronicum Migrans | Borreliosis | Chirwere cheLyme | Chirwere Chekutanga | Chikumi 2011 | Chikamu chechina |
| NCT02538224 | Islamic Azad University, Tehran | Chisingaperi Periodontitis | Chikunguru 2013 | Chikamu 2 | Phase 3 |
| NCT00066027 | Yunivhesiti yeNebraska | National Institute of Dental uye Craniofacial Research (NIDCR) | Periodontitis | Chikumi 2002 | Phase 3 |
| NCT00376493 | Hospital de Clinicas de Porto Alegre | Septic Abortion | Chivabvu 2006 | Chikamu chechina |
| NCT03448731 | Fundacion CRIS de Investigación ye Vencer el Cáncer|Amgen|Apices Soluciones SL | Skin Toxicity | Chivabvu 10, 2018 | Phase 2 |
| NCT00989742 | Yunivhesiti yeNottingham | Lymphangioleiomyomatosis | Tuberous Sclerosis | Chikunguru 2009 | Chikamu chechina |
| NCT01438515 | Horizon Health Network | Methicillin-resistant Staphylococcus Aureus | Nyamavhuvhu 2008 | Hazvigoneke |
| NCT02929121 | The Task Force for Global Health|United States Agency for International Development (USAID) | Lymphedema | Lymphatic Filariasis | Filariasis | Ndira 15, 2019 | Phase 3 |
| NCT00952861 | Odense University Hospital|Kolding Sygehus|Svendborg Hospital|Fredericia Hospital|Naestved Hospital|Hillerod Hospital, Denmark|Region Syddanmark|Danmarks Lungeforening|Danish National Research Foundation | Pulmonary Disease, Chronic obstructive | Gumiguru 2009 | Chikamu chechina |
| NCT00138801 | Sorlandet Hospital HF | Lyme Neuroborreliosis | Kurume 2004 | Phase 3 |
| NCT00942006 | Yunivhesiti Medical Center Ljubljana | Kufungidzirwa Kwekutanga Lyme Neuroborreliosis | Chikunguru 2009 | Hazvigoneke |
| NCT02713607 | Yunivhesiti yeCalifornia, Davis | Acne Vulgaris | Kurume 2016 | Chikamu 1 | Chikamu 2 |
| NCT00560703 | Galderma | Blepharitis | Meibomianitis | Ziso Rakaoma | Mbudzi 2007 | Phase 2 |
| NCT01014260 | Johns Hopkins University | Cardiovascular Disease | Gunyana 2010 | Chikamu chechina |
| NCT00000938 | National Institute of Allergy uye Infectious Diseases (NIAID) | Chirwere cheLyme | Phase 3 | |
| NCT01398072 | University College, London|Royal Yemahara Hampstead NHS Trust|Yunivhesiti yeCambridge|National Institute for Health Research, United Kingdom | Chronic Obstructive Pulmonary Disease (COPD). | Zvita 2011 | Phase 3 |
| NCT03479502 | Vanderbilt University Medical Center | Orthopedic Research uye Dzidzo Foundation | Adhesive Capsulitis | Adhesive Capsulitis yeMapfudzi Asingazivikanwi | Ndira 5, 2018 | Chikamu chechina |
| NCT02929134 | The Task Force for Global Health|United States Agency for International Development (USAID) | Lymphedema | Lymphatic Filariasis | Filariasis | Kukadzi 16, 2018 | Phase 3 |
| NCT00480532 | Oregon Health uye Science University | Kudzivirira pamuviri, Nemuromo | Chivabvu 2007 | Hazvigoneke |
| NCT01594827 | Johns Hopkins University | Nyaya yeWestern Reserve University | Cystic Fibrosis Foundation | Cystic Fibrosis | Gumiguru 2012 | Phase 2 |
| NCT01744093 | Weill Medical College yeCornell University | National Heart, Lung, uye Ropa Institute (NHLBI) | HIV | Chronic Obstructive Pulmonary Disease (COPD)| Emphysema | Chikunguru 17, 2014 | Hazvigoneke |
| NCT03530319 | National Taiwan University Hospital | Pneumonia, Mycoplasma | Mbudzi 10, 2018 | Hazvigoneke |
| NCT04167085 | Yunivhesiti yeCalifornia, Los Angeles | Epistaxis | Zvita 18, 2017 | Chikamu chechina |
| NCT01411202 | Ottawa Hospital Research Institute | Inokuvadza Pleural Effusion | Chikumi 2011 | Phase 2 |
| NCT01474590 | Galderma | Mburwa | Mbudzi 2011 | Phase 3 |
| NCT00649571 | Nhoroondo yeMylan Pharmaceuticals | Hutano | Chikunguru 2005 | Phase 1 |
| NCT02899000 | Galderma Laboratories, LP | Acne Vulgaris | Chikunguru 29, 2016 | Chikamu chechina |
| NCT00538967 | Leiden University Medical Center | Aortic Aneurysm, Mudumbu | Chivabvu 2002 | Phase 2 |
| NCT00439400 | Nhoroondo ye Alacrity Biosciences, Inc. | Ziso Rakaoma | Kukadzi 2007 | Phase 2 |
| NCT00917553 | Thomas Gardner|Penn State University|Vadiki Diabetes Research Foundation|Milton S. Hershey Medical Center | Chirwere cheshuga retinopathy | Chikunguru 2009 | Phase 2 |
| NCT00495313 | CollaGenex Pharmaceuticals | Rosacea | Kurume 2007 | Chikamu chechina |
| NCT01855360 | Brigham uye Chipatara chevakadzi | Amyloidosis; Mwoyo (Kuratidza)|Senile Cardiac Amyloidosis | Chikumi 2013 | Chikamu 1 | Chikamu 2 |
| NCT00419848 | Shahid Beheshti University of Medical Sayenzi | Mburwa | Nyamavhuvhu 2006 | Phase 2 |
| NCT03532464 | University Hospital, Bordeaux|USC EA 3671 Infections humanes à mycoplasmes uye chlamydiae | Chlamydia Trachomatis Infection | Vaginal Infection | Anal Infection | Chikunguru 1, 2018 | Chikamu chechina |
| NCT02756403 | Medstar Health Research Institute | Sangano reKuronga Mhuri | First Trimester Kubvisa pamuviri | Kurume 2016 | Hazvigoneke |
| NCT00353158 | National Institute of Arthritis uye Musculoskeletal uye Skin Zvirwere (NIAMS)|National Institutes of Health Clinical Center (CC) | Vazvipiri Vane Utano | Fungal Infections| Utachiona hweBhakitiriya | Chikunguru 11, 2006 | Phase 1 |
| NCT01317433 | Institut Cancerology de l'Ouest | Colorectal Cancer Metastatic | Ganda Toxicity | Zvita 2010 | Phase 3 |
| NCT01658995 | Petra M. Casey|Mayo Clinic | Kubuda ropa kwakabatana neESI | Gunyana 13, 2012 | Phase 3 |
| NCT03968562 | State University yeNew York - Downstate Medical Center | Mikoko | Chivabvu 15, 2019 | Phase 2 |
| NCT02569437 | Icahn Chikoro cheMishonga paGomo reSinai | Polyp yeNasal Sinus | Gunyana 2014 | Phase 2 |
| NCT01198509 | NYU Langone Hutano|National Institute of Arthritis uye Musculoskeletal uye Skin Zvirwere (NIAMS)|Chirangaridzo Sloan Kettering Cancer Center | Rheumatoid Arthritis | Psoriatic Arthritis | Periodontal Disease | Ndira 2010 | Hazvigoneke |
| NCT01163994 | Yunivhesiti Medical Center Ljubljana | Multiple Erythema Migrans | Chikumi 2010 | Hazvigoneke |
| NCT02388477 | Milton S. Hershey Medical Center | Rotator Cuff Kukuvara | Hazvigoneke | |
| NCT01010295 | International Extranodal Lymphoma Study Group (IELSG) | Non-Hodgkin Lymphoma | Gunyana 2006 | Phase 2 |
| NCT00775918 | Ranbaxy Laboratories Limited | Ranbaxy Inc. | Hutano | Chikumi 2005 | Hazvigoneke |
| NCT04050540 | University of Washington|Kenya Medical Research Institute|Kenya National AIDS & STI Control Programme|University of California, San Francisco|National Institute of Allergy and Infectious Diseases (NIAID) | Utachiona hweHIV|HIV+AIDS|Neisseria Gonorrheae Infection|Chlamydia Trachomatis Infection|Syphilis Infection | Kukadzi 5, 2020 | Chikamu chechina |
| NCT02562651 | Russian Academy of Medical Sayenzi | Zvirwere zveVascular | Cardiovascular Diseases | Acute Myocardial Infarction | Kukadzi 2014 | Chikamu 2 | Phase 3 |
| NCT00001101 | National Institute of Allergy uye Infectious Diseases (NIAID) | Chirwere cheLyme | Phase 3 | |
| NCT00340691 | National Institute of Allergy and Infectious Diseases (NIAID)|National Institutes of Health Clinical Center (CC) | Mansonella Perstans Infection|Mp Microfilaremia | Zvita 6, 2004 | Phase 2 |
| NCT01112059 | Yunivhesiti yeAlabama kuBirmingham | Cystic Fibrosis Foundation | Cystic Fibrosis | Mbudzi 2008 | Hazvigoneke |
| NCT00652704 | Par Pharmaceutical, Inc.|Anapharm | Kuti usarudze Bioequivalence pasi peFed Conditions | Chikunguru 1999 | Phase 1 |
| NCT01783860 | Tehran Yunivhesiti yeMedical Sayenzi | Posterior Blepharitis | Ndira 2013 | Phase 2 |
| NCT02564471 | State University yeNew York - Upstate Medical University|Walter Reed Army Institute of Research (WRAIR)|Kansas State University | Chimbwamupengo | Kubvumbi 2016 | Chikamu chechina |
| NCT04206631 | Indonesia University | Acne Vulgaris | Kubvumbi 1, 2015 | Phase 1 |
| NCT03956446 | University Medical Center Ljubljana|Yunivhesiti yeLjubljana Chikoro cheMishonga, Slovenia | Chikwekwe Borne Encephalitis | Gunyana 1, 2014 | Hazvigoneke |
| NCT03960411 | Felix Chikita Fredy, MD|National Cardiovascular Center Harapan Kita Hospital Indonesia|Indonesia University | ST Elevation Myocardial Infarction|Anterior Wall Myocardial Infarction|Kutadza Kwemoyo|Kugadziridza, Ventricular | Chivabvu 25, 2019 | Phase 3 |
| NCT00322465 | National Institute of Allergy uye Infectious Diseases (NIAID) | Urethritis | Mbudzi 2006 | Phase 2 |
| NCT01375491 | University of California, San Diego|Ruth L. Kirschstein National Research Service Award|National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)|National Center for Research Resources (NCRR) | Type 2 Diabetes|Kufutisa | Gumiguru 2009 | Chikamu chechina |
| NCT03478436 | Medical University yeVienna | Dr. Iyo kambani Reddy's Laboratories Limited | Rosacea | Chikunguru 2016 | Phase 1 |
| NCT01207739 | Radboud University|Sint Maartenskliniek|ZonMw: Sangano reNetherlands reKutsvaga Hutano neBudiriro | Chirwere cheLyme | Borrelia Infection | Gunyana 2010 | Chikamu chechina |
| NCT00939562 | Pfizer | Utachiona hweBhakitiriya | Mbudzi 2008 | Chikamu chechina |
| NCT03608774 | National Institute of Allergy uye Infectious Diseases (NIAID) | Anal Chlamydia Infection | Chikumi 26, 2018 | Chikamu chechina |
| NCT02281643 | Kwame Nkrumah Yunivhesiti yeScience uye Technology | Yunivhesiti yeBonn | Heinrich-Heine University, Duesseldorf | Mansonella Perstans Infection|Buruli Ulcer|Tuberculosis|Co-infection | Gumiguru 2014 | Phase 2 |
| NCT00066066 | Iyo Forsyth Institute | National Institute of Dental uye Craniofacial Research (NIDCR) | Periodontitis | Zvirwere zvePeriodontal | Chikunguru 2003 | Phase 2 |
| NCT01798225 | Medical University yeSouth Carolina | National Center for Research Resources (NCRR) | Periodontal Disease | Type 2 Diabetes Mellitus | Zvita 2007 | Chikamu chechina |
| NCT00612573 | Warner Chilcott | Acne Vulgaris | Kukadzi 2008 | Phase 2 |
| NCT01631617 | National Institute of Arthritis uye Musculoskeletal uye Skin Zvirwere (NIAMS)|National Institutes of Health Clinical Center (CC) | Eczema|Dermatitis|Zvirwere zveganda, Genetic|Dermatitis, Atopic|Zvirwere zveganda | Gunyana 18, 2012 | Phase 2 |
| NCT03173053 | Radboud University|ZonMw: The Netherlands Organisation for Health Research and Development|Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA)|Aalborg University Hospital|Rigshospitalet, Denmark | Staphylococcus Aureus | Motility Disorder | Kukadzi 8, 2018 | Hazvigoneke |
| NCT00715858 | McMaster University | The Physicians' Services Incorporated Foundation | Chirwere cheAlzheimer | Chivabvu 2008 | Phase 3 |
| NCT03584919 | Yunivhesiti Medical Center Ljubljana | Erythema Chronicum Migrans | Chikumi 1, 2006 | Hazvigoneke |
| NCT01469585 | Yunivhesiti yeHawaii | Charles Drew University yeMishonga neSainzi | Meharry Medical College | Breakthrough Bleeding | Mbudzi 2011 | Hazvigoneke |
| NCT02759120 | Weill Medical College yeCornell University|Duke Clinical Research Institute|Yunivhesiti yeChicago|Yunivhesiti yeWashington|Yunivhesiti yePittsburgh|National Heart, Lung, uye Ropa Institute (NHLBI) | Idiopathic Pulmonary Fibrosis | Kurume 22, 2017 | Phase 3 |
| NCT02735837 | Amirhossein Farahmand|Islamic Azad University, Tehran | Diabetes Mellitus With Periodontal Disease | Ndira 2015 | Chikamu 2 | Phase 3 |
| NCT03655197 | Yunivhesiti yeCalifornia, Davis | Rosacea | Ocular Rosacea | Cutaneous Rosacea | Mbudzi 2, 2017 | Early Phase 1 |
| NCT01188954 | Northwell Health | Seroma | Ndira 2010 | Hazvigoneke |
| NCT00388778 | Shahid Beheshti University of Medical Sayenzi | Acne|Kuzvimba | Gumiguru 2005 | Chikamu 2 | Phase 3 |
| NCT01087476 | Metropolitan Autonomous University | Instituto Nacional yeCancerology yeMexico | Mucositis | Chivabvu 2010 | Phase 2 |
| NCT02174757 | CD Pharma India Pvt. Ltd.|Sree Mookambika Institute of Dental Sciences | Chisingaperi Periodontitis | Nyamavhuvhu 2014 | Phase 3 |
| NCT03911440 | National Taiwan University Hospital | Atypical Pneumonia | Mbudzi 10, 2018 | Hazvigoneke |
| NCT02553083 | Rabin Medical Center | Utachiona hweBhakitiriya nekuda kweHelicobacter Pylori (H. Pylori) | Gumiguru 22, 2015 | Chikamu chechina |
| NCT04234945 | Ahmadu Bello University Teaching Hospital | Kusabereka, Mukadzi | Pelvic Inflammatory Disease | Ndira 13, 2020 | Hazvigoneke |
| NCT00892281 | Galderma Laboratories, LP | Rosacea | Kubvumbi 2009 | Chikamu chechina |
| NCT02913118 | Qingfeng Pharmaceutical Group | Community Yakawana Pneumonia | Chikunguru 2016 | Chikamu chechina |
| NCT04153604 | Methodist Health System | Cirrhosis | Kungoerekana kwaita Bhakitiriya Peritonitis | November 4, 2019 |
|
| NCT03153267 | University Medical Center Ljubljana|Yunivhesiti yeLjubljana Chikoro cheMishonga, Slovenia | Erythema Chronicum Migrans | Chikumi 1, 2017 | Hazvigoneke |
| NCT03116659 | James J. Peters Veterans Affairs Medical Center | Lymphoma, T-Cell, Cutaneous | Kukadzi 1, 2018 | Early Phase 1 |
| NCT03401372 | Jian Li|Peking University First Hospital|Chinese PLA General Hospital|Beijing Chao Yang Hospital|West China Hospital Yakabatana neSichuan University|Tongji Hospital Yakabatana neTongji Medical College yeHUST|Union Hospital Yakabatana neTongji Medical College yeHUST|Shanghai Changzheng Hospital| Nanfang Hospital yeSouthern Medical University | Peking Union Medical College Hospital | Amyloidosis; Systemic | Kubvumbi 21, 2018 | Hazvigoneke |
| NCT01380496 | Par Pharmaceutical, Inc.|Anapharm | Kuti usarudze Bioequivalence pasi peFed Conditions | Mbudzi 1999 | Phase 1 |
| NCT03083197 | University of Oxford|Shoklo Malaria Research Unit|Chiangrai Prachanukroh Hospital | Kukwesha Typhus | Gumiguru 15, 2017 | Chikamu chechina |
| NCT00237016 | Medical Corps, Israel Defence Force | Kudzokorora Fever, Tick-Borne|Jarisch Herxheimer Reaction | Kubvumbi 2002 | Chikamu 2 | Phase 3 |
| NCT01308619 | Galderma Laboratories, LP | Rosacea | Kubvumbi 2011 | Chikamu chechina |
| NCT01198912 | Chipatara cheYunivhesiti, Ghent | Chronic Rhinosinusitis | Nasal Polyps | Mbudzi 22, 2011 | Phase 2 |
| NCT02016365 | Umeå University | Transthyretin Amyloidosis | Cardiomyopathy | Kukadzi 2012 | Phase 2 |
| NCT00783523 | Yunivhesiti yeCalifornia, San Francisco | Arteriovenous Malformations|Cavernous Angiomas|Brain Aneurysms | Kurume 2008 | Phase 1 |
| NCT03337932 | Yunivhesiti Medical Center Ljubljana | Erythema Chronicum Migrans | Ndira 1, 2018 | Hazvigoneke |
| NCT00568711 | Dong-Min Kim | Chosun University Hospital | Kukwesha Typhus | Gunyana 2006 | Hazvigoneke |
| NCT01874860 | Yunivhesiti yeLouisville | James Graham Brown Cancer Center | Colorectal Cancer | Musoro uye Mutsipa Kenza | Nyamavhuvhu 2013 | Phase 2 |
| NCT01171859 | IRCCS Policlinico S. Matteo | Transthyretin Amyloidosis | Chikunguru 2010 | Phase 2 |
| NCT01653522 | Iyo Cleveland Clinic | Migraine Disorders|Musoro, Migraine|Migraine|Migraine Headache|Migraine With Aura|Migraine Pasina Aura|Musoro Unorwadza, Primary | Chikunguru 2012 | Hazvigoneke |
| NCT01820910 | International Extranodal Lymphoma Study Group (IELSG) | Marginal Zone Lymphoma yeOcular Adnexal | Kurume 2013 | Phase 2 |
| NCT01323101 | Yunivhesiti yeSouthern California | Cystic Fibrosis | Kubvumbi 2008 | Chikamu chechina |
| NCT00829764 | Teva Pharmaceuticals USA | Hutano | Gumiguru 2006 | Phase 1 |
| NCT01668498 | AIO-Studien-gGmbH | Ras-wildtype Colorectal Cancer | Chivabvu 2011 | Phase 2 |
| NCT01030666 | Peter Eickholz|Heidelberg University|Dr. August Wolff GmbH & Co. KG Arzneimittel|Gaba International AG|Goethe University | Periodontitis | Kubvumbi 2007 | Chikamu chechina |
| NCT00012688 | US Department of Veterans Affairs|Colgate-Periogard-Dentsply|VA Hofisi Yekutsvagisa nebudiriro | Chirwere cheshuga mellitus | Yakashata Glycemic Control | Peridontal Chirwere | Hazvigoneke | |
| NCT01885910 | Derm Research, PLLC | WFH MEDICAL, LLC | Acne Vulgaris | Chikunguru 2013 | Chikamu chechina |
| NCT02328469 | University Medical Center Ljubljana|Slovenian Research Agency|Yunivhesiti yeLjubljana Chikoro cheMishonga, Slovenia|Harvard University | Aseptic Meningitis | Chikumi 2014 |
|
| NCT00355602 | Yunivhesiti yeDundee | Tenovus Scotland | Colitis, Zvironda | Chikunguru 2006 | Hazvigoneke |
| NCT02606032 | Hamilton Hutano Sayenzi Corporation | Hamilton Academic Health Sayenzi Sangano | Ulcerative Colitis | Chivabvu 2016 | Phase 2 |
| NCT01465802 | Pfizer | Isiri Diki Cell Lung Cancer (NSCLC) | Zvita 26, 2011 | Phase 2 |
| NCT02623959 | MD Anderson Cancer Center | Zvirwere zveCancer | Zvakaipa Pleural Effusions | Kubvumbi 27, 2016 | Chikamu chechina |
| NCT03481972 | IRCCS Policlinico S. Matteo | TTR Mwoyo Amyloidosis | Kubvumbi 11, 2018 | Phase 3 |
| NCT00428818 | Yunivhesiti yeTexas Southwestern Medical Center | Utachiona | Nyamavhuvhu 2005 | Hazvigoneke |
| NCT01935622 | Virginia Commonwealth University | Non-ischemic Cardiomyopathy|Systolic Heart Failure (NYHA II-III) | Chikunguru 2012 | Phase 2 |
| NCT01886560 | Sun Yat-sen University | Kupisa Kwemaziso | Gunyana 2013 | Chikamu 2 | Phase 3 |
| NCT04239755 | Damanhour University | Tanta University | Traumatic Brain Injury | Zvita 15, 2019 | Chikamu chechina |
| NCT02204254 | Center Hospitaler Universitaire de Nice | Rosacea | Kurume 2014 | Hazvigoneke |
| NCT00837213 | Stiefel, Kambani yeGSK|GlaxoSmithKline | Mburwa | Nyamavhuvhu 2007 | Chikamu chechina |
| NCT03115177 | Rush University Medical Center | Osteoarthritis | Mbudzi 2015 | Hazvigoneke |
| NCT03618108 | Cadrock Pty. Ltd.|Center for Digestive Diseases, Australia | Chirwere cheMwoyo cheCoronary | Chlamydophila Pneumoniae Infections | Kubvumbi 4, 2018 | Phase 2 |
| NCT03435952 | MD Anderson Cancer Center|BioMed Valley Discoveries, Inc|Merck Sharp & Dohme Corp. | Neoplasm yezamu Sites|Akaipa Neoplasms ye Lip Oral Cavity uye Pharynx| Huru Neoplasms yeMarume Genital Organs | Inokuvadza Neoplasms yeMesothelial uye Soft Tissue | Inokuvadza Neoplasms yeKufema uye Intrathoracic Organs | Inokuvadza Neoplasms yeThroid uye Mamwe Endocrine Glands | Maturakiti Akashata | Chikunguru 10, 2018 | Phase 1 |
| NCT01867294 | Yedzidzo uye Yenharaunda Cancer Research United | National Cancer Institute (NCI) | Yepamusoro Malignant Neoplasm | Dermatologic Complication | Nyamavhuvhu 31, 2012 | Phase 2 |
| NCT01677286 | Boston University | Amyloidosis | Chikunguru 2012 | Phase 2 |
| NCT00511875 | Thomas Gardner|Vadiki Diabetes Research Foundation|Milton S. Hershey Medical Center | Chirwere cheshuga retinopathy | Chikunguru 2008 | Phase 2 |
| NCT04108897 | Johns Hopkins University | Rosacea | Gunyana 17, 2019 | Early Phase 1 |
| NCT00631501 | Kaunas University of Medicine | Chipatara cheYunivhesiti, Linkoeping | Lateral Epicondylalgia (Tennis Elbow) | Hazvigoneke | |
| NCT02203682 | Sun Yat-sen University | Graves Ophthalmopathy| Zvirwere zveMakuva| Zvirwere zveZiso | Zvirwere zveThyroid | Endocrine System Zvirwere | Chikunguru 2014 | Phase 2 |
| NCT02005653 | Indian Council of Medical Research | Filarial; Infestation | Kukadzi 2009 | Chikamu chechina |
| NCT03585140 | Centro Dermatológico Dr. Ladislao de la Pascua | Acne Vulgaris | Kugadziriswa Kwekudya | Ndira 1, 2016 | Hazvigoneke |
| NCT02147262 | University Medical Center Ljubljana|Yunivhesiti yeLjubljana Chikoro cheMishonga, Slovenia|Medical University yeVienna|Harvard University | Chronic Atrophic Acrodermatitis | Chikunguru 2013 | Hazvigoneke |
| NCT02220751 | Yunivhesiti yeSao Paulo|Fundação de Amparo à Pesquisa do Estado de São Paulo | Periodontitis | Type 2 Diabetes Mellitus | Kurume 2009 | Phase 3 |
| NCT01825408 | Yunivhesiti yeNorth Carolina, Chapel Hill | Sinusitis | Kukadzi 2013 | Chikamu chechina |
| NCT02884713 | King Faisal Specialist Hospital & Research Center | GASTRITIS | Chikumi 2013 | Hazvigoneke |
| NCT02726646 | Yunivhesiti yeCampinas, Brazil|Pontificia Universidade Catolica de Sao Paulo | Chisingaperi Periodontitis | Chikumi 2015 | Phase 2 |
| NCT00883818 | Samsung Medical Center | Overactive Bladder | Ndira 2007 | Chikamu chechina |
| NCT00829790 | Teva Pharmaceuticals USA | Hutano | Gumiguru 2006 | Phase 1 |
| NCT01949233 | Yunivhesiti yeOxford | Oxford University Hospitals NHS Trust | Marfan Syndrome | Gumiguru 2013 | Phase 2 |
| NCT01518192 | University Medical Center Ljubljana|Slovenian Research Agency | Erythema Migrans | Post-Lyme Chirwere Zviratidzo | Chikumi 2006 | Chikamu chechina |
| NCT02845024 | Islamic Azad University, Tehran | Diabetes Mellitus With Periodontal Disease | Gunyana 2014 | Hazvigoneke |
| NCT01879930 | University Hospital Inselspital, Berne | Chronic Pelvic Pain Syndrome | Dundira Pain Syndrome | Mbudzi 2012 | Chikamu chechina |
| NCT00041977 | CollaGenex Pharmaceuticals | Acne Rosacea | Chikumi 2002 | Phase 3 |
| NCT02341209 | Rochester General Hospital | Cutaneous T-cell Lymphoma | Mycosis Fungoides | Sezary Syndrome | Kukadzi 6, 2018 | Phase 2 |
| NCT00002872 | Eastern Cooperative Oncology Group|National Cancer Institute (NCI)|North Central Cancer Treatment Boka | Metastatic Cancer | Mbudzi 1996 | Phase 3 |
| NCT03162497 | Medical University yeVienna | Dry Eye Syndromes|Meibomian Gland Dysfunction | Ndira 8, 2018 | Chikamu chechina |
| NCT01418742 | Gesellschaft fur Medizinische Innovation ? Hamatologie und Onkologie mbH|ClinAssess GmbH | Colorectal Carcinoma | Nyamavhuvhu 2011 | Phase 2 |
| NCT00980148 | National Institute of Allergy uye Infectious Diseases (NIAID) | Chlamydial Infection | Zvita 2009 | Phase 3 |
| NCT03342456 | The Third Xiangya Hospital yeCentral South University|Livzon Pharmaceutical Group Inc.|Yung Shin Pharm. Ind. Co., Ltd. | Duodenal Ulcer Nokuda kweHelicobacter Pylori | Zvita 13, 2017 | Chikamu chechina |
| NCT04310930 | Yunivhesiti yeQueensland|Dhipatimendi reHurumende yeAustralia yehutano|Chipatara chevana Foundation|Cystic Fibrosis Foundation|Newcastle University|Griffith University|Erasmus Medical Center|Monash University|University of Copenhagen|Hôpital Cochin|South Australian Health and Medical Research Institute|Yunivhesiti yeMelbourne|James Cook University, Queensland, Australia|Murdoch Childrens Research Institute | Chirwere chePulmonary nekuda kweMycobacteria (Kuongororwa) | Kurume 2020 | Chikamu 2 | Phase 3 |
| NCT03709459 | Kirby Institute|South Australia Hutano uye Medical Research Institute|Monash University | Kudzivirira STIs | Zvita 17, 2019 |
|
| NCT04067011 | Emergent BioSolutions|Biomedical Advanced Research and Development Authority | Chigwadara | Nyamavhuvhu 12, 2019 | Phase 2 |
| NCT02844634 | British Columbia Center for Disease Control | HIV|Syphilis | Chivabvu 15, 2018 | Chikamu chechina |
| NCT00647959 | Nhoroondo yeMylan Pharmaceuticals | Hutano | Kurume 2006 | Phase 1 |
| NCT00170222 | Medical Center Alkmaar | Chronic Obstructive Pulmonary Disease | Chikunguru 2002 | Chikamu chechina |
| NCT03075891 | Galderma | Rosacea | Chikunguru 5, 2017 | Chikamu chechina |
| NCT00031499 | National Institute of Allergy uye Infectious Diseases (NIAID) | Syphilis | June 2000 | Phase 3 |
| NCT01205464 | Linkoeping University | Kuneta|Kurwadza Kusingaite | Kukadzi 2005 | Hazvigoneke |
| NCT01301586 | Nhoroondo ye Nexgen Dermatologics, Inc. | ACNE VULGARIS | Mbudzi 2010 | Chikamu 1 | Chikamu 2 |
| NCT02305940 | Imperial College London | Chirwere Chisingaperi chePulmonary Disease (COPD) | Chikunguru 2014 | Phase 3 |
| NCT00351182 | Dong-Min Kim | Chosun University Hospital | Kukwesha Typhus | Gunyana 2005 | Phase 3 |
| NCT03334682 | Nantes University Hospital | Acne Vulgaris | Ndira 31, 2018 | Phase 3 |
| NCT01788215 | Yunivhesiti yeRochester | Polycystic Ovarian Syndrome (PCOS)|Kusakwana Kwekuenda Kumwedzi | Androgen Yakawandisa | Mbudzi 2010 | Phase 3 |
| NCT03076281 | Sidney Kimmel Cancer Center paThomas Jefferson University | Thomas Jefferson University | Larynx|LIP|Oral Cavity|Pharynx | Kubvumbi 3, 2017 | Phase 2 |
| NCT00439166 | Hamilton Health Sciences Corporation | The Physicians' Services Incorporated Foundation|McMaster University | Chirwere cheAlzheimer | Kukadzi 2007 | Phase 3 |
| NCT02463942 | University Medical Center Ljubljana|Yunivhesiti yeLjubljana Chikoro cheMishonga, Slovenia | Chikwekwe Encephalitis | Gunyana 2014 | Hazvigoneke |
| NCT00803842 | Northwestern University | Non Diki Cell Lung Cancer | Gumiguru 2008 | Hazvigoneke |
| NCT02086591 | Yunivhesiti yeRochester | Munhu mukuru Diffuse Large B-Cell Lymphoma|Mantle Cell Lymphoma Recurrent|Lymphoma, Follicular|Marginal Zone B-Cell Lymphoma|Malignant Lymphoma - Lymphoplasmacytic|Waldenstrom Macroglobulinemia|Small Lymphocytic Lymphoma|Chronic Lymphocytic Leukemia-Cll) | Kurume 2014 | Phase 2 |
| NCT03980223 | Yunivhesiti yeCalifornia, San Francisco | Yunivhesiti yeWashington | National Institute of Allergy uye Infectious Diseases (NIAID) | Mayne Pharma International Pty Ltd | San Francisco Dhipatimendi reHutano Hwevanhu. | Gonorrhea | Chlamydia | Syphilis | Mbudzi 26, 2019 | Chikamu chechina |
| NCT00355459 | Yunivhesiti yeTexas Southwestern Medical Center | Dry Eye Syndrome | Nyamavhuvhu 2005 | Hazvigoneke |
| NCT01254799 | Omar Mamdouh Shaaban|Assiut University | Uterine Hemorrhage | Ndira 2008 | Phase 3 |
| NCT01547325 | NanoSHIFT LLC|United States Dhipatimendi reDziviriro | Dehisced Kuvhiya Maronda | Chivabvu 2012 | Hazvigoneke |
| NCT00653380 | Par Pharmaceutical, Inc.|Anapharm | Kuona Bioequivalence Pasi peKutsanya Mamiriro | Gunyana 1999 | Phase 1 |
| NCT00635609 | Warner Chilcott | Acne Vulgaris | Kurume 2008 | Chikamu chechina |
| NCT03765931 | Institut de Recherche pour le Developpement | Kupindwa nechando | Chikunguru 2016 | Chikamu chechina |
| NCT01160640 | Harold Wiesenfeld | National Institute of Allergy uye Infectious Diseases (NIAID) | Yunivhesiti yePittsburgh | Pelvic Inflammatory Disease | Mbudzi 2010 | Phase 2 |
| NCT01756833 | Yunivhesiti yeMaryland, Baltimore | National Institute on Aging (NIA) | Aneurysm | Chivabvu 2013 | Phase 2 |
| NCT00688064 | Galderma | Yakanyanya Acne Vulgaris | Nyamavhuvhu 2008 | Phase 3 |
| NCT01320033 | Galderma | Acne Vulgaris | Kurume 29, 2011 | Phase 2 |
| NCT03397004 | St. Michael's Hospital, Toronto|Barrow Neurological Institute|Duke University|Feinstein Institute for Medical Research|Yunivhesiti yePittsburgh|Sunnybrook Health Sciences Center | Hereditary Hemorrhagic Telangiectasia (HHT) | Gunyana 12, 2018 | Phase 2 |
| NCT01635530 | Turku University Hospital | Lyme Neuroborreliosis | Nyamavhuvhu 2012 | Chikamu chechina |
| NCT03727620 | Mohammed V Souissi University | Aggressive Periodontitis | Ndira 6, 2014 | Chikamu 1 | Chikamu 2 |
| NCT02688738 | Rothman Institute Orthopedics | Propionibacterium | Kurume 2015 | Hazvigoneke |
| NCT00358462 | Yunivhesiti yeWashington | National Institute of Allergy uye Infectious Diseases (NIAID) | Urethritis | Ndira 2007 | Phase 3 |
| NCT02864550 | British Columbia Center for Disease Control | Syphilis | Utachiona Hwepabonde | Nyamavhuvhu 15, 2019 | Chikamu chechina |
| NCT01595594 | Yunivhesiti yeSao Paulo|Fundação de Amparo à Pesquisa do Estado de São Paulo | Periodontal Disease | Type 2 Diabetes | Kurume 2010 | Phase 3 |
| NCT00964834 | PharmAthene, Inc.|National Institutes of Health (NIH)|Medarex|Quintiles, Inc.|Department of Health and Human Services | Chigwadara | Chikunguru 2009 | Phase 1 |
| NCT01809444 | Sun Yat-sen University | Thyroid Associated Opthalmopathies | Mbudzi 2012 | Chikamu 2 | Phase 3 |
| NCT01590082 | MD Anderson Cancer Center | National Institutes of Health (NIH) | National Cancer Institute (NCI) | Melanoma | Mbudzi 2012 | Chikamu 1 | Chikamu 2 |
| NCT00207584 | Centers for Disease Control and Prevention | Mycoplasma Pneumoniae | Ndira 1994 | Hazvigoneke |
| NCT00775177 | Ranbaxy Laboratories Limited | Ranbaxy Inc. | Hutano | Chikumi 2005 | Hazvigoneke |
| NCT03462329 | Yunivhesiti Medical Center Ljubljana | Erythema Migrans | Chikumi 1, 2018 | Hazvigoneke |
| NCT00000403 | Indiana University | National Institute of Arthritis uye Musculoskeletal uye Skin Zvirwere (NIAMS) | National Institute on Aging (NIA) | Osteoarthritis | Gunyana 1996 | Phase 3 |
| NCT03508232 | Yunivhesiti yeAlberta | Royal Alexandra Hospital | ST Segment Elevation Myocardial Infarction|Kutadza Kwemoyo | Ndira 6, 2020 | Phase 2 |
| NCT02553473 | Sorlandet Hospital HF | Neuroborreliosis, Borrelia Burgdorferi | Gumiguru 2015 | Phase 3 |
| NCT02207556 | Medical College yeWisconsin | Primary Systemic Amyloidosis | Gumiguru 1, 2014 | Phase 2 |
| NCT01783106 | Royal Liverpool University Hospital | National Association for Colitis uye Crohn's Chirwere | National Institute for Health Research, United Kingdom | Chirwere cheCrohn | Kukadzi 1, 2014 | Phase 2 |
| NCT00353743 | Hospital de Clinicas de Porto Alegre | Kubvisa pamuviri, Septic | Chivabvu 2006 | Hazvigoneke |
| NCT01727973 | Sun Yat-sen University | Graves Ophthalmopathy| Zvirwere zveMakuva| Zvirwere zveZiso | Zvirwere zveThyroid | Endocrine System Zvirwere | Gumiguru 2012 | Chikamu 1 | Chikamu 2 |
| NCT00857038 | Medical Center Alkmaar|Leiden University Medical Center|Yunivhesiti yeAmsterdam | Chirwere Chisingaperi Chinodzivirira Pulmonary Disease|Kuzvimba | Pulmonary Emphysema | Kubvumbi 2009 | Chikamu chechina |
| NCT02774993 | National University Hospital, Singapore|Tan Tock Seng Hospital|National University, Singapore|A*Star | Tibhii | Gunyana 2015 | Phase 2 |
| NCT03474458 | IRCCS Policlinico S. Matteo | Cardiac AL Amyloidosis | Kukadzi 11, 2019 | Chikamu 2 | Phase 3 |
| NCT02874430 | Sidney Kimmel Cancer Center paThomas Jefferson University | Thomas Jefferson University | Breast Carcinoma|Endometrial Clear Cell Adenocarcinoma|Endometrial Serous Adenocarcinoma|Uterine Corpus Cancer|Uterine Corpus Carcinosarcoma | Chikumi 8, 2016 | Phase 2 |
| NCT00016835 | Yunivhesiti yeMichigan | National Institute of Dental uye Craniofacial Research (NIDCR) | Periodontal Chirwere | Chirwere cheshuga mellitus, Rudzi rwechipiri | Gumiguru 17, 2001 | Phase 2 |
| NCT00064766 | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) | Endometrial Bleeding | Periodontal Disease | Kukadzi 2003 | Chikamu chechina |
| NCT00803452 | Yunivhesiti yeLouisville | Blepharitis | Chikunguru 2008 | Chikamu chechina |
| NCT01434173 | Bayer|RTI Health Solutions | Kukuvara Kwechiropa Kunokonzerwa Nezvinodhaka | Chikunguru 2001 |
|
| NCT00126204 | Barnes-Jewish Hospital | Aortic Aneurysm | Kurume 2004 | Hazvigoneke |
| NCT01917721 | Hawaii Pacific Health | Chirwere cheKawasaki | Coronary Aneurysm | Gumiguru 2013 | Phase 2 |
| NCT02775695 | Medical College yeWisconsin | Resectable Pancreatic Cancer | Kubvumbi 3, 2017 | Phase 2 |
| NCT03824340 | Chipatara cheAljazeera | Kushaya mbereko | Ndira 30, 2019 | Hazvigoneke |
| NCT01847976 | Ottawa Hospital Research Institute | Canadian Breast Cancer Foundation | Marwadzo | Nyamavhuvhu 2013 | Phase 2 |
| NCT02850913 | Makerere University | Yunivhesiti yeOxford | Kubatwa nepfari | Gunyana 5, 2016 | Phase 2 |
| NCT00764361 | NanoSHIFT LLC | Diabetic Foot Ulcer | Ndira 2009 | Phase 2 |
| NCT02036528 | Nhoroondo ye Royer Biomedical, Inc. | Diabetic Foot Ulcers | Ndira 2014 | Chikamu 1 | Chikamu 2 |
| NCT01661985 | Ostergotland County Council, Sweden|Statens Serum Institut | Urethritis|cervicitis|Genital Mycoplasma Infection|Chlamydia Trachomatis | Kukadzi 2010 | Chikamu chechina |
| NCT01380483 | Par Pharmaceutical, Inc.|Anapharm | Kuona Bioequivalence Pasi peKutsanya Mamiriro | Ndira 2000 | Phase 1 |
| NCT00648180 | Nhoroondo yeMylan Pharmaceuticals | Hutano | Chikunguru 2005 | Phase 1 |
| NCT01426269 | Galderma Laboratories, LP | Rosacea | Gunyana 2011 | Chikamu chechina |
| NCT02753426 | Yunivhesiti yeCalifornia, San Francisco | Chirwere cheKidney Chisingaperi | Cardiorenal Syndrome | Kubvumbi 2016 | Phase 1 |
| NCT02583282 | Postgraduate Institute of Medical Dzidzo uye Tsvagiridzo | Inokuvadza Pleural Effusion | Nyamavhuvhu 1, 2015 | Hazvigoneke |
| NCT02927496 | The Task Force for Global Health|United States Agency for International Development (USAID) | Lymphedema | Lymphatic Filariasis | Filariasis | Chikumi 19, 2018 | Phase 3 |
| NCT00652795 | Par Pharmaceutical, Inc.|Anapharm | Kuona Bioequivalence Pasi peKutsanya Mamiriro | Chikunguru 2004 | Phase 1 |
| NCT03956212 | University Medical Center Ljubljana|Yunivhesiti yeLjubljana Chikoro cheMishonga, Slovenia | Erythema Migrans | Chikumi 1, 2017 | Hazvigoneke |
| NCT00855595 | Bayer | Papulopustular Rosacea | Kukadzi 2009 | Chikamu chechina |
| NCT03457636 | Derm Research, PLLC | Mburwa | Kurume 19, 2018 | Chikamu chechina |
| NCT02894268 | Sir Run Run Shaw Hospital | Helicobacter Pylori Infection | Kukadzi 2016 | Chikamu chechina |
| NCT03465774 | MD Anderson Cancer Center | National Cancer Institute (NCI) | Inokuvadza Pleural Effusion | Kurume 8, 2018 | Early Phase 1 |
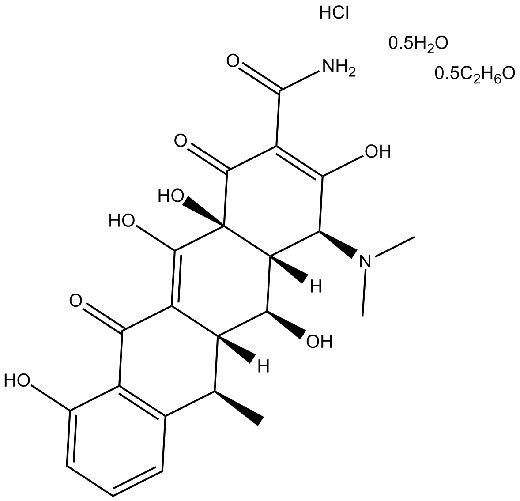
Chimiro chemakemikari





Proposal18Quality Consistency Evaluation mapurojekiti akatenderwa4,uye6mapurojekiti ari pasi pekubvumidzwa.

Yepamberi yepasirese yemhando manejimendi system yakaisa hwaro hwakasimba hwekutengesa.

Kutariswa kwemhando yepamusoro kunomhanya kuburikidza nehupenyu hwese kutenderera kwechigadzirwa kuti ive nechokwadi chemhando uye yekurapa maitiro.

Professional Regulatory Affairs timu inotsigira zvinodiwa zvemhando panguva yekushandisa uye kunyoreswa.


Korea Countec Bottled Packaging Line


Taiwan CVC Bottled Packaging Line


Italy CAM Board Packaging Line

German Fette Compacting Machine

Japan Viswill Tablet Detector

DCS Control Room







