Niraparib 1038915-60-4
Tsanangudzo
Niraparib (MK-4827) ine simba zvakanyanya uye nemuromo bioavailable PARP1 uye PARP2 inhibitor ine IC50s ye3.8 uye 2.1 nM, zvichiteerana.Niraparib inotungamira mukudzivirirwa kwekugadzirisa kwekukuvara kweDNA, inomutsa apoptosis uye inoratidza anti-bundu chiitiko.
In Vitro
Niraparib (MK-4827) inhibisa PARP chiitiko neEC50=4 nM uye EC90=45 nM mune yesero yesero assay.MK-4827 inhibisa kuwanda kwekenza maseru ane mutant BRCA-1 uye BRCA-2 ine CC50 mu10-100 nM renji.MK-4827 inoratidza yakanakisa PARP 1 uye 2 inhibition ine IC50 = 3.8 uye 2.1 nM, zvichiteerana, uye mune yesero yesero assay[1].Kusimbisa kuti Niraparib (MK-4827) inhibits PARP mumasero aya, A549 uye H1299 masero anobatwa ne1.μM MK-4827 kwenguva dzakasiyana uye yakayera PARP enzymatic chiitiko uchishandisa chemiluminescent assay.Zvigumisiro zvinoratidza kuti Niraparib (MK-4827) inodzivisa PARP mukati memaminitsi e15 ekurapa anosvika anenge 85% inhibition mumasero eA549 pa1 h uye inenge 55% inhibition pa 1 h yeH1299 masero.
Niraparib (MK-4827) inoregererwa zvakanaka uye inoratidza kushanda senge mumiriri mumwechete mune xenograft modhi yeBRCA-1 isina kukwana cancer.Niraparib (MK-4827) inoshivirirwa zvakanaka mu vivo uye inoratidza kushanda semumiriri mumwechete mune xenograft modhi yeBRCA-1 isina kukwana cancer.Niraparib (MK-4827) inoratidzirwa ne pharmacokinetics inogamuchirwa mumakonzo ane plasma clearance ye28 (mL/min)/kg, yakakwira kwazvo kugovera (Vd).ss= 6.9 L/kg), refu terminal hafu yeupenyu (t1/2=3.4 h), uye yakanakisa bioavailability, F=65%[1].Niraparib (MK-4827) inosimudzira mhinduro yemwaranzi yep53 mutant Calu-6 tumor mune ese ari maviri, ine imwechete zuva rega rega ye50 mg/kg ichinyanya kushanda kupfuura 25 mg/kg inopiwa kaviri zuva nezuva.].
Storage
| Upfu | -20°C | 3 years |
| 4°C | 2 years | |
| In solvent | -80°C | 6 mwedzi |
| -20°C | 1 mwedzi |
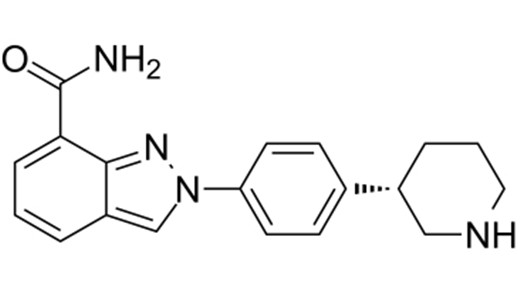
Chimiro chemakemikari





Proposal18Quality Consistency Evaluation mapurojekiti akatenderwa4,uye6mapurojekiti ari pasi pekubvumidzwa.

Yepamberi yepasirese yemhando manejimendi system yakaisa hwaro hwakasimba hwekutengesa.

Kutariswa kwemhando yepamusoro kunofamba kuburikidza nehupenyu hwese kutenderera kwechigadzirwa kuti ive nechokwadi chemhando nekurapa.

Professional Regulatory Affairs timu inotsigira zvinodiwa zvemhando panguva yekushandisa uye kunyoreswa.


Korea Countec Bottled Packaging Line


Taiwan CVC Bottled Packaging Line


Italy CAM Board Packaging Line

German Fette Compacting Machine

Japan Viswill Tablet Detector

DCS Control Room




