Ribociclib 1374639-75-4
Tsanangudzo
Ribociclib (LEE01) ndeye zvakanyanya kujeka CDK4/6 inhibitor ine IC50 kukosha kwegumi nM uye 39 nM, zvichiteerana, uye inopfuura chiuru-yakapetwa isina simba kurwisa cyclin B/CDK1 yakaoma.
In Vitro
Kurapa mapaneru e17 neuroblastoma cell mitsara neRibociclib (LEE011) pane mana-log dose renji (10 kusvika 10,000 nM).Kurapa neRibociclib kunovharisa zvakanyanya substrate adherent kukura maererano nekutonga mu12 ye17 neuroblastoma cell mitsara yakaongororwa (zvinoreva IC50=306±68 nM, tichifunga mitsara yakaoma chete, apo kunzwa kunotsanangurwa seIC50 isingasviki 1.μM. Ribociclib kurapwa kweviri neuroblastoma cell lines (BE2C uye IMR5) nekuratidzira kunzwisiswa kuCDK4/6 inhibition inoguma mukuunganidzwa-kunoenderana nedosi kuunganidzwa kwemasero muG0/G1 chikamu chesero cycle.Uku kusungwa kweG0/G1 kunova kwakakosha paRibociclib concentration ye100 nM (p=0.007) uye 250 nM (p=0.01), zvichiteerana.
CB17 immunodeficient mice dzine BE2C, NB-1643 (MYCN amplified, sensitive in vitro), kana EBC1 (isina-amplified, resistant in vitro) xenografts inorapwa kamwe chete zuva nezuva kwemazuva 21 neRibociclib (LEE011; 200 mg/kg) kana ne kutonga kwemotokari.Iri dosing zano rinotenderwa zvakanaka, sezvo pasina kurasikirwa nehuremu kana zvimwe zviratidzo zvechepfu zvinoonekwa mune chero yemhando dzexenograft.Kukura kwebundu kunonyanya kunonoka mukati memazuva makumi maviri nerimwe ekurapwa mumakonzo ane BE2C kana 1643 xenografts (zvose, p <0.0001), kunyange zvazvo kukura kwakatangazve mushure mekurapa.
Storage
| Upfu | -20°C | 3 years |
| 4°C | 2 years | |
| In solvent | -80°C | 6 mwedzi |
| -20°C | 1 mwedzi |
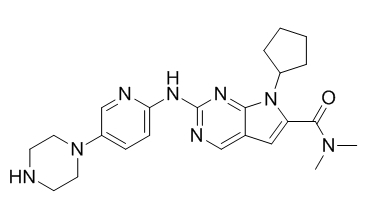
Chimiro chemakemikari





Proposal18Quality Consistency Evaluation mapurojekiti akatenderwa4,uye6mapurojekiti ari pasi pekubvumidzwa.

Yepamberi yepasirese yemhando manejimendi system yakaisa hwaro hwakasimba hwekutengesa.

Kutariswa kwemhando yepamusoro kunofamba kuburikidza nehupenyu hwese kutenderera kwechigadzirwa kuti ive nechokwadi chemhando nekurapa.

Professional Regulatory Affairs timu inotsigira zvinodiwa zvemhando panguva yekushandisa uye kunyoreswa.


Korea Countec Bottled Packaging Line


Taiwan CVC Bottled Packaging Line


Italy CAM Board Packaging Line

German Fette Compacting Machine

Japan Viswill Tablet Detector

DCS Control Room










